In January 2021, Alcon launched a new intraocular lens implant (IOL) called “Vivity”. Vivity is described as a “first-of-its-kind”, non-diffractive, extended depth of focus intraocular lens implant. The lens received FDA approval in February 2020 and is now widely available to patients across the United States. It is available in a toric lens implant version so that patients with corneal astigmatism can have it.
In this article, we’re going to give you a Vivity lens review. You’ll learn all the details of the Vivity IOL, such as how it works, and the results you can expect from it. Let’s get started!
What Does “Extended Depth of Focus” Mean?
“Extended depth of focus IOL’s” are designed to give patients a broader range of vision than “monofocal” implants that have a single focal point, and are typically aimed for either distance or near vision. “Multifocal implants” have multiple focal points, and can be thought of as bifocal or trifocal implants.
As an example, consider a bifocal implant, such as the ZMB00 Multifocal implant made by Johnson & Johnson. The ZMB00 is designed to give excellent distance vision, and excellent reading vision at approximately 13 inches. However, intermediate-range vision, which is typically where a computer monitor or the dashboard in the car sits, may be blurry. The “sweet spots” of vision with this type of lens do not have a large range. For example, a patient with the ZMB00 may have excellent reading vision at 13 inches, but when they move the object just a few inches forward or backward, it quickly becomes blurry.
“Extended depth of focus” means that instead of having a relatively small sweet spot of clear vision, the lens is designed to ideally give patients a larger range of useful vision.
What Makes The Design Of The Vivity IOL Different?
One of the side effects of many Multifocal IOL’s (MFIOL’s) is glare and halo symptoms. The benefit of many Multifocal IOL’s is achieved by “diffracting” light. Essentially this means “scattering light” in a controlled way. As light enters the eye “diffractive MFIOL’s” split this light into the distance and near focal points for distance and near vision. As a side effect, some of this scattered light is perceived as glare, halos, and a reduction in the sharpness of vision. Different MFIOL’s cause different amounts of glare and halo symptoms. If you are interested in MFIOL’s, this is a conversation to have with your surgeon.
The Vivity was designed to reduce glare and halo symptoms as much as possible compared to other MFIOL’s on the market. This lens may be an option for diabetics with cataracts, however, this should be based on a discussion with your surgeon, since there is a broad spectrum of severity of diabetic eye diseases.
As a trade-off, the Vivity does not provide as close of near vision as some other MFIOL’s. The Vivity is targeted more for the “intermediate” range of vision (approximately 26 inches), which would be more suited for computer and dashboard range vision than the near reading of small print.
How Does The Vivity IOL Perform?
Next in our Vivity lens review, let’s discuss how the Vivity IOL performs. A clinical trial for the Vivity IOL studied 220 patients, with 107 receiving the Vivity IOL, and 113 receiving a monofocal control IOL at 11 sites in the United States. The average age of patients in the study was 68 years old. One patient from the Vivity group did not receive the Vivity in their 2nd eye and was not included in the analysis.
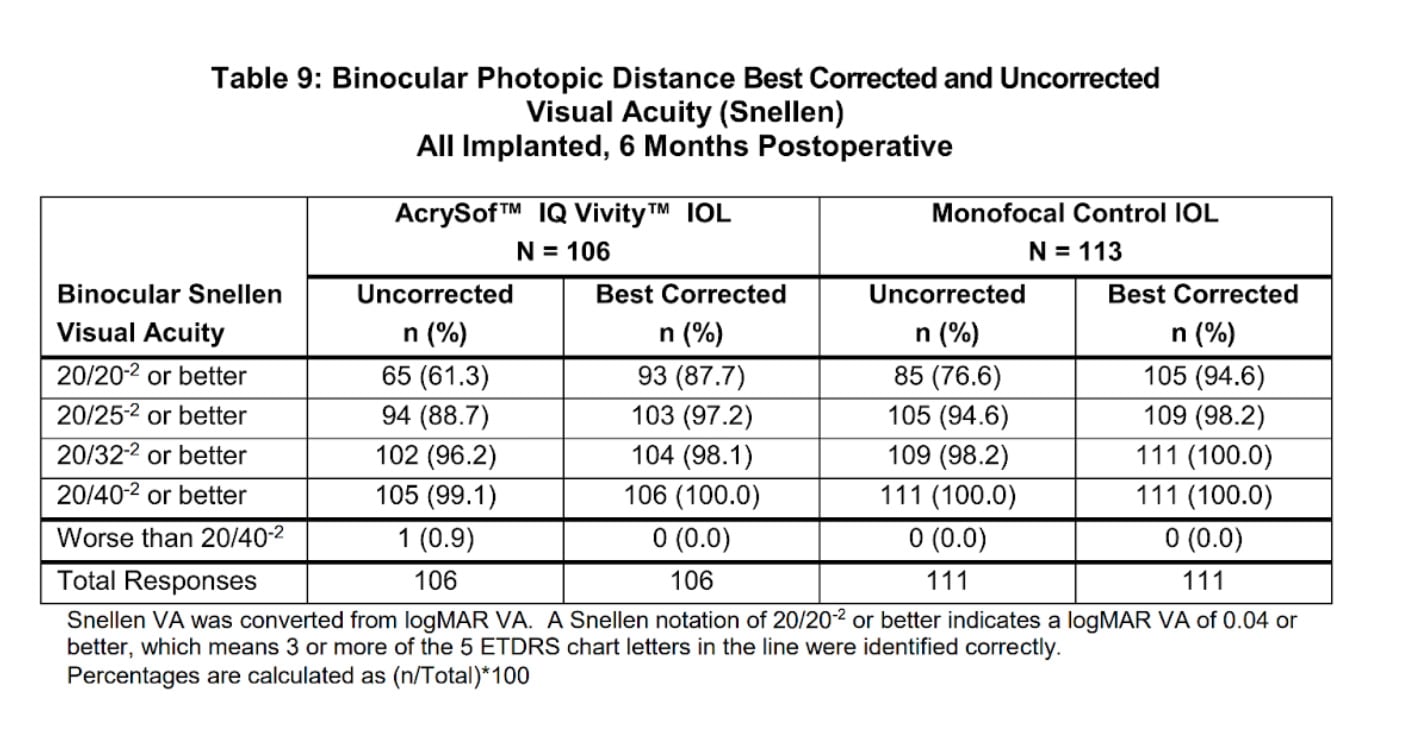
Vivity Lens Distance Vision
Let’s first take a look at how patients’ Distance vision compared after surgery between the Vivity IOL and the standard monofocal control IOL:
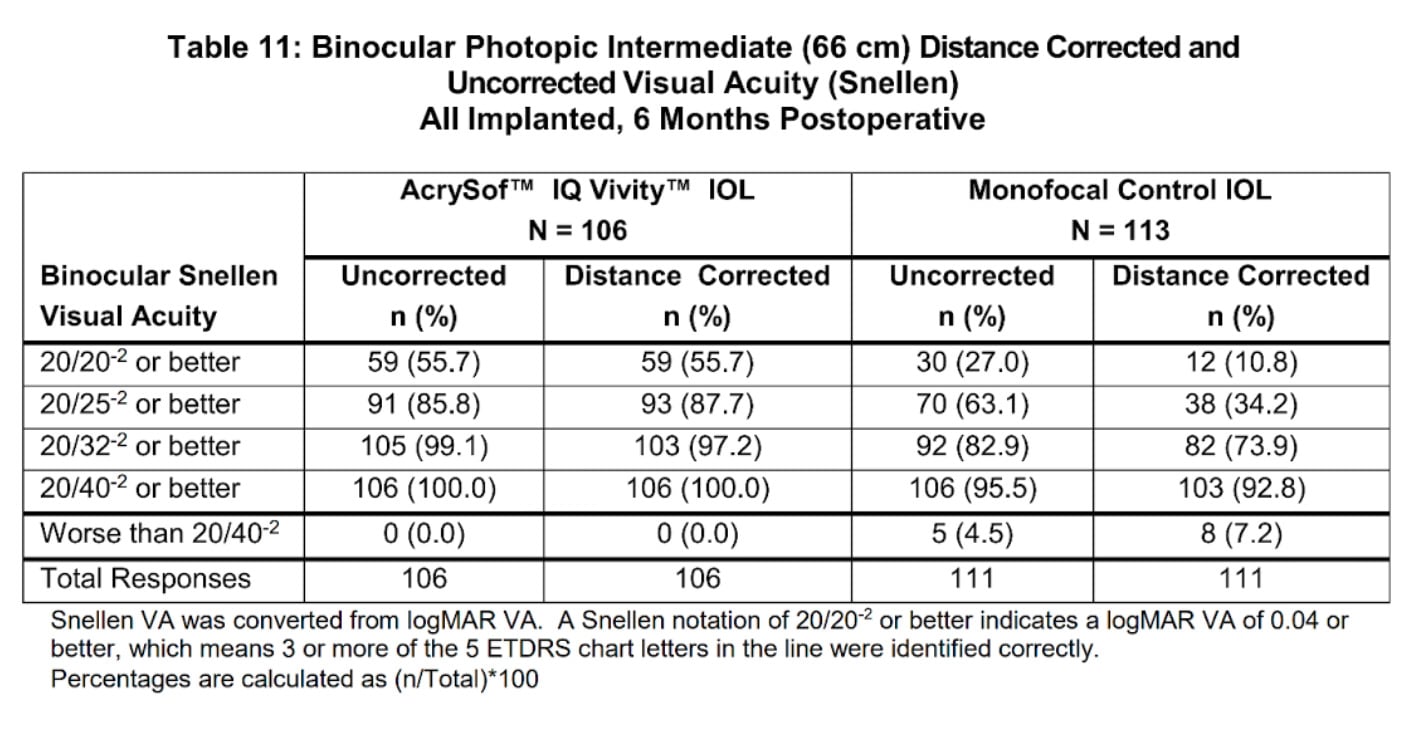
Vivity Lens Near Vision (Really “Intermediate” Vision)
Let’s next take a look at how the Vivity compared to the monofocal control lens for Intermediate range vision, which is defined as 66 centimeters from the eye:
Vivity Lens Side Effects
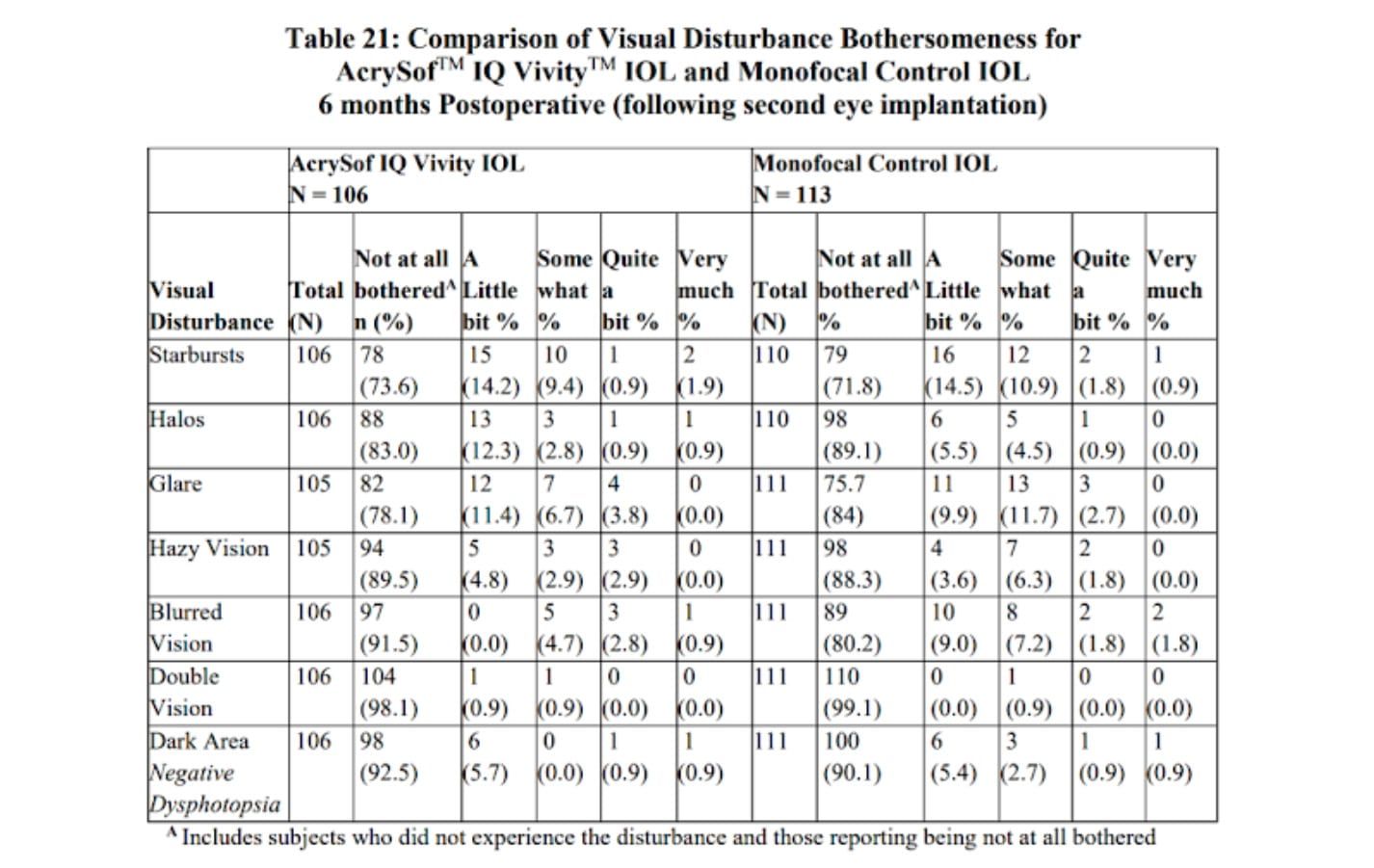
Let’s look at the side effects of the Vivity lens implant such as glare and halos next… We would expect the Monofocal control to have a minimal amount of complaints of glare and halos. The goal of the Vivity is to achieve its “extended depth of focus” without significantly increasing glare/halos symptoms compared to the monofocal IOL:
Finally, let’s look at functional outcomes… In other words, most patients would choose the Vivity IOL with the goal of decreasing their dependence on glasses in mind… so let’s look at how patients reported their need for glasses after surgery for distance, intermediate, and near vision:
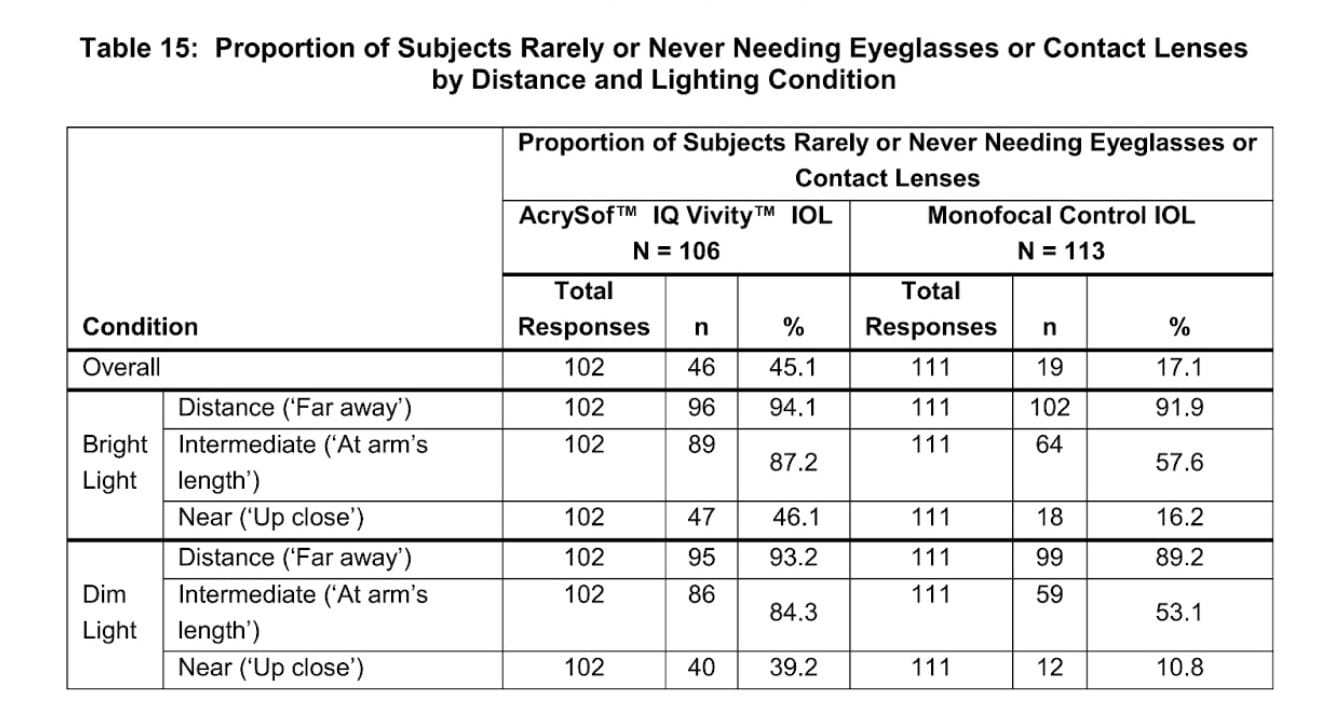
(Note: The study asked patients about their need for glasses in both bright light and dim light conditions. One reason for this is that, in addition to the brightness of light illuminating objects being viewed, pupil size is also different in light versus dark conditions.)
Vivity Lens Patient Reviews / Testimonials
Let’s take a look at a few Vivity lens patient reviews to hear about their experience with the Vivity IOL (Videos provided by Alcon):
Cost of Vivity Lens
Medical insurance does not entirely cover the cost of cataract surgery with the Vivity IOL. Medical insurance will cover part of the surgery, but not all of it, and there will be an out-of-pocket expense.
Overall Summary Of The Vivity IOL
The Vivity is an excellent lens and will make for a lot of happy patients… However, you’ll hear us say this a lot when it comes to implants…. There is no “best” or “one-size-fits-all” lens implant option. All IOL’s come with a different profile of trade-offs, or risks & benefits. While the Vivity performed better than the Monofocal IOL for Intermediate range vision, it was not 100% effective in achieving complete independence from glasses at this range. There also was a slight worsening of distance vision with the Vivity compared to the Monofocal IOL. This can be thought of as a trade-off of being willing to give up a slight amount of distance vision (which many patients may not even notice) for the benefit of gaining intermediate range vision.
Overall, there are many nuances to selecting the right IOL for your lifestyle.
Sources
- 1. Alcon Vivity IOL “Directions for Use”
- 2. FDA Summary of Safety and Effectiveness Data (SSED) for the AcrySof™ IQ Vivity Extended Vision Intraocular Lens (IOL) (Model DFT015), AcrySof™ IQ Vivity™ Toric Extended Vision IOLs (DFT315, DFT415, DFT515), AcrySof™ IQ Vivity™ Extended Vision UV Absorbing IOL (DAT015), and AcrySof™ IQ Vivity™ Toric Extended Vision UV Absorbing IOLs (DAT315, DAT415, DAT515) – P930014/S126. Available June 2021.
- 3. Videos courtesy of Alcon.
lllᐅ DIWUJI 3 Pieces Fitness Elastic Bands, 1.5M Elastic Resistance Bands Exercise Set with 3 Resistance Levels for Yoga, Pilates, Crossfit, Strength Stretching – Fitness Web proscalpin shape up fitness – 118 w byrd blvd universal city, tx – health & fitness, exercise & fitness programs – (210)-908-9595